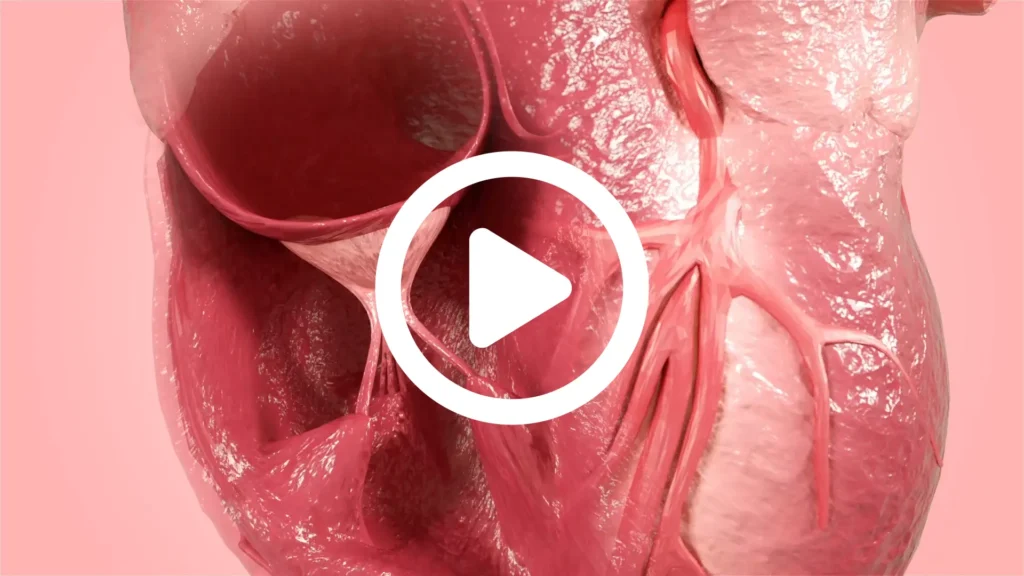
Products
CorPatch® Epicardial Repair
Indications For Use:
CorPatch® is intended for epicardial tissue support and repair.
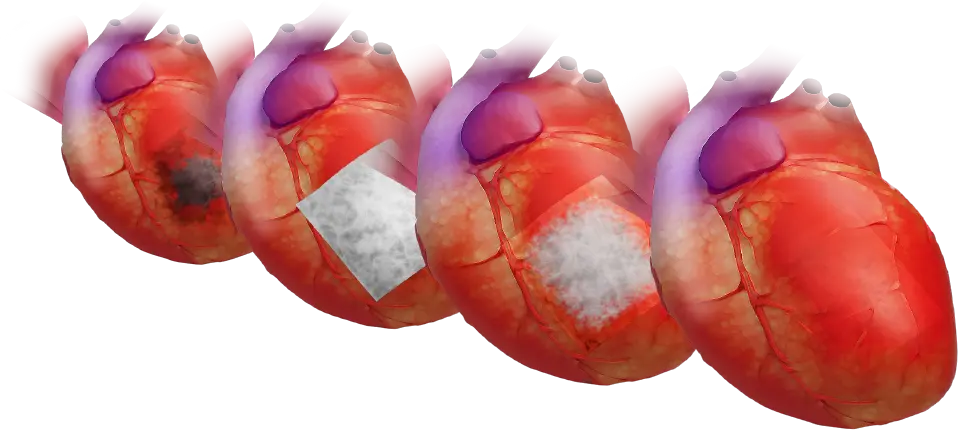
- CorPatch acts as a decellularized ECM® (extracellular matrix) scaffold for supporting repair of weak tissue during cardiac surgery
- CorPatch ECM® scaffold allows the patient’s own cells to migrate and attach within the ECM to naturally support and rebuild weakened cardiac tissue
Epicardial Support and Repair
Product Information
CorPatch®, Epicardial Patch 5 PACK
- 5cm x 5cm: CMCP5P-055
- 7cm x 10cm: CMCP5P-710
Pipeline
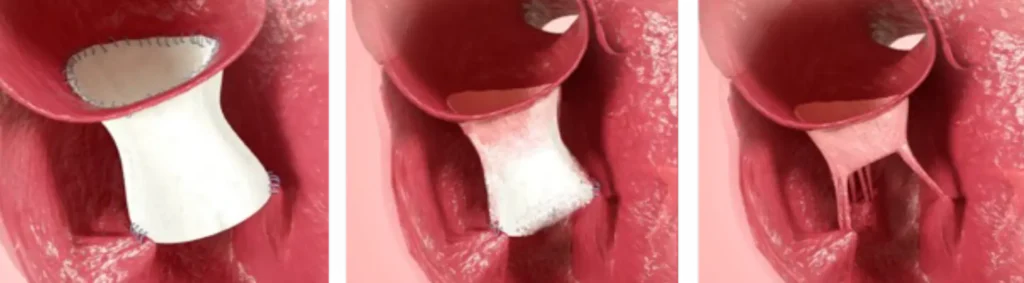
CorTricuspid ECM® Valve
With potential to:
- Replace, regenerate and restore tricuspid valve
- Match native valve dimensions with various size options
- Encourage vessel regeneration to revascularize tissue
- Rebuild tissue by incorporating patient’s own cells
Cardiac Valve Replacement and Regeneration
CorMatrix® CorTricuspid ECM® Pivotal Study
CorMatrix CorTricuspid ECM Valve Study is designed to demonstrate the clinical safety and performance of the CorTricuspid ECM Valve for the surgical management of tricuspid valve disease and dysfunction in adult and pediatric subjects with TR.
Up to 110 subjects at up to 15 study sites will undergo tricuspid valve replacement (TVR) with the CorTricuspid ECM Valve for the surgical management of tricuspid valve disease or dysfunction.
News
CorMatrix® News
- CorMatrix® Cardiovascular Receives FDA Approval for Landmark Pivotal Trial for a Regenerative Heart Valve | Learn More
CorMatrix® Events
- CorMatrix® Presentation | View it Now
- KOL Webinar on a Novel Approach to Cardiovascular Treatment: CorMatrix’s ECM Technology | View it Now
About
CorMatrix® Cardiovascular
CorMatrix is the world leader in cardiovascular regeneration, addressing the largest challenges in cardiovascular disease, including valvular heart disease, structural heart disorders and myocardial infarction.
It is developing the CorTricuspid ECM® Valve for the surgical management of tricuspid valve disease and dysfunction in adult and pediatric subjects.
The company is also expanding its epicardial repair program with its FDA cleared CorPatch® epicardial repair product. CorPatch® is a second-generation extracellular matrix device engineered for epicardial placement for support and repair of weakened areas of the heart after myocardial infarction.
Tricuspid Valve Regurgitation (TR)
TR is a type of valvular heart disease or dysfunction in which the tricuspid valve of the heart does not close completely, allowing blood to flow backwards from the right ventricle to the right atrium. As severity increases, TR can lead to lower quality of life and increase mortality.
CorMatrix® Cardiovascular Leaders

John P. Engels, MBA
CEO
- Successful business, operations, and commercial leader
- Deep knowledge of biologic, ECM device and regulations
- History of successful capital raises and company exits

Robert Matheny, M.D.
Co-Founder, CTO
- Expertise in regenerative medicine, and cardiac surgery
- Strong surgical expertise and KOL relationships
- Numerous patents, inventions and product launches